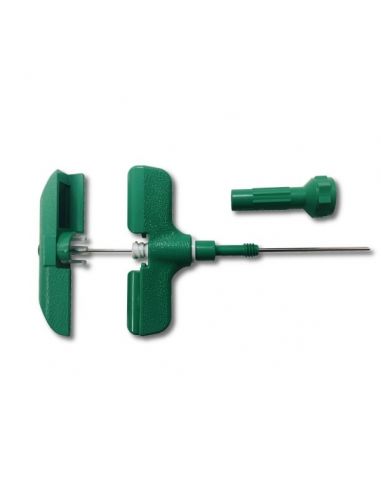
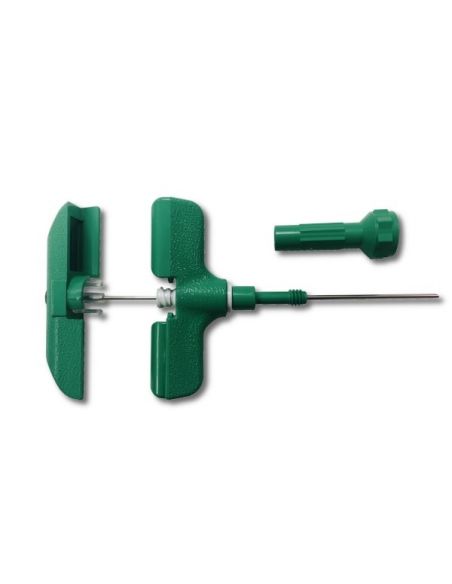
Bone marrow aspiration needle 15g x 10,2cm max. (box 10) Luer lock connector on the handle
Bone marrow aspiration needle 15G x 10,2cm max. (box 10)
This "15G x 10,2cm max" aspiration needle is indicated for the bone marrow aspiration in the sternum or iliac crest.
Safe aspirations at the sternum can be performed by adjusting the needle stop to control the depth of penetration.
This needle stop can be removed if aspirating at the iliac crest.
Product Benefits :
? Luer lock connector on the handle for secure syringe attachement
? An adjustable, removable needle stop safely controls depth of penetration
? Can be used via the iliac pathway by removing the adjustable stop
Device Composition :
? Stylet (Stainless steel)
? Cannula (Stainless steel)
? Stylet hub (ABS plastic)
? Cannula hub (Polycarbonate)
? Sheath (LDPE)
Additional Informations:
? Medical device sterilized with ethylene oxide
? Single use medical device
? No latex
? No phthalates (DHP)
Precaution :
? Check the integrity of the sterility protector before use.
? Use before the expiration date.
? Store in a cool and dry place.
? Do not re-sterilize: single use.
? Comply with the instructions for use.
Packaging:
? Box of 10 units
The needles are delivered with a non-stick cannula guide to facilitate the alignment of the cannula in the needle tip.
This protects the tip to eliminate the risks of accidental injury.
This product is CE certified.
This "15G x 10,2cm max" aspiration needle is indicated for the bone marrow aspiration in the sternum or iliac crest.
Safe aspirations at the sternum can be performed by adjusting the needle stop to control the depth of penetration.
This needle stop can be removed if aspirating at the iliac crest.
Product Benefits :
? Luer lock connector on the handle for secure syringe attachement
? An adjustable, removable needle stop safely controls depth of penetration
? Can be used via the iliac pathway by removing the adjustable stop
Device Composition :
? Stylet (Stainless steel)
? Cannula (Stainless steel)
? Stylet hub (ABS plastic)
? Cannula hub (Polycarbonate)
? Sheath (LDPE)
Additional Informations:
? Medical device sterilized with ethylene oxide
? Single use medical device
? No latex
? No phthalates (DHP)
Precaution :
? Check the integrity of the sterility protector before use.
? Use before the expiration date.
? Store in a cool and dry place.
? Do not re-sterilize: single use.
? Comply with the instructions for use.
Packaging:
? Box of 10 units
The needles are delivered with a non-stick cannula guide to facilitate the alignment of the cannula in the needle tip.
This protects the tip to eliminate the risks of accidental injury.
This product is CE certified.
- Material
- Acier inoxydable
- Gauge / French
- 15G
- Length mm
- 102
- Net weight kg
- 0,50
- Packing
- 23x20x12cm
- Gross weight kg
- 0,60
- Interventional procedure
- Biopsie ostéo médulaire (BOM)
- Sterile
- Stérile EO
- CE
- DM Classe IIa
101379
6 other products in the same category: